43 fda guidance use of symbols on labels
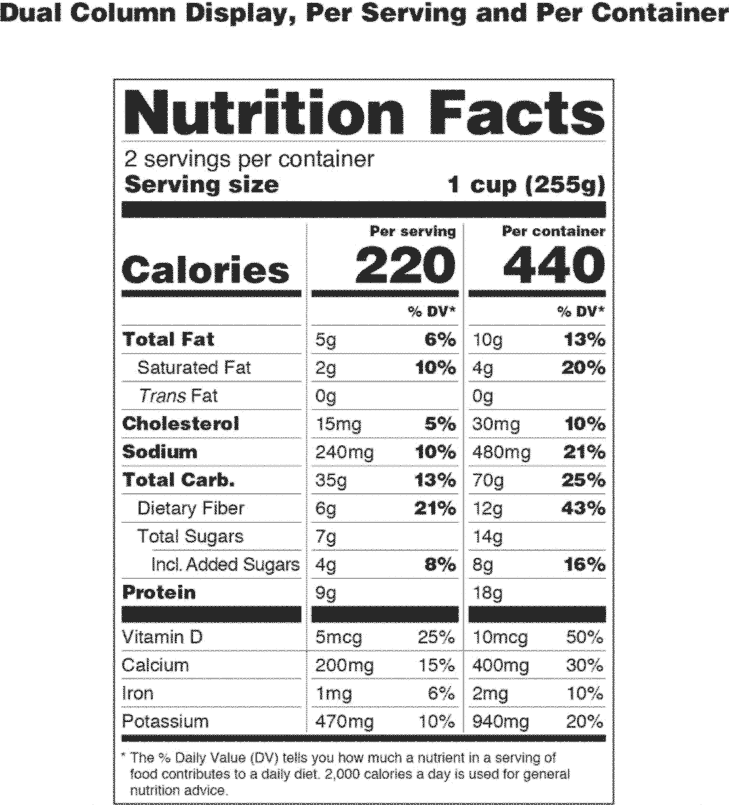
CPG Sec.140.500 Metric Declarations of Quantity of Contents ... In support of this policy, the agency has developed the following guidance on the use of the metric system in declaring the net quantity of contents on the labels of FDA-regulated commodities. Use of the Term Healthy on Food Labeling | FDA Oct 07, 2022 · The FDA has begun a public process to update the "healthy" claim for food labeling to be consistent with current nutrition science and federal dietary guidance.
510(K) Submissions for Piston Syringes Guidance The submission shall contain proposed labels, labeling, and advertisements sufficient to describe the device, its intended use, and the directions for use. Labels include the information affixed ...

Fda guidance use of symbols on labels
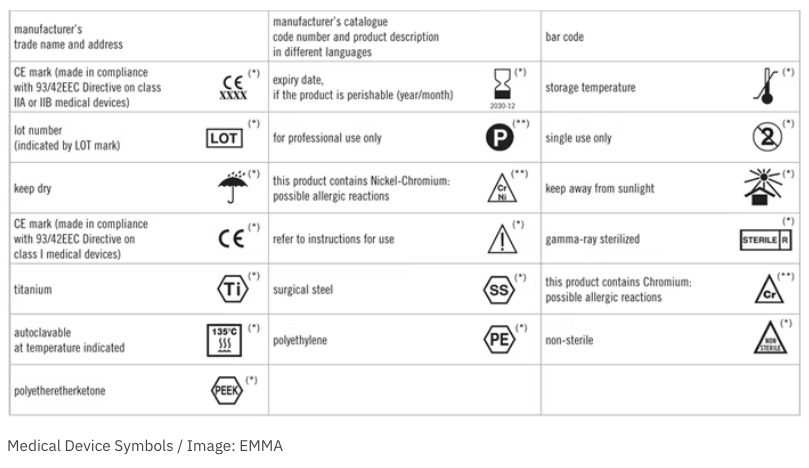
Use of Symbols in Labeling: Frequently Asked Questions | FDA Manufacturers should look to the final rule, not the withdrawn 2004 guidance, when determining their use of symbols in new labeling and when making labeling updates. 14. CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ... Labeling & Nutrition Guidance Documents & Regulatory ... Guidance for Industry: Converting Units of Measure for Folate, Niacin, and Vitamins A, D, and E on the Nutrition and Supplement Facts Labels August 2019; Guidance for Industry: Declaration of ...
Fda guidance use of symbols on labels. Small Entity Compliance Guide on Structure/Function Claims |FDA This is a Level 2 guidance document published for immediate implementation in accordance with FDA's good guidance practices (21 CFR 10.115). ... medical symbols on labels? ... which the use of ... Labeling & Nutrition Guidance Documents & Regulatory ... Guidance for Industry: Converting Units of Measure for Folate, Niacin, and Vitamins A, D, and E on the Nutrition and Supplement Facts Labels August 2019; Guidance for Industry: Declaration of ... CFR - Code of Federal Regulations Title 21 - Food and Drug ... Jul 20, 2022 · (4) The meaning or explanatory text for the symbol as provided in the FDA recognition or, if FDA has not recognized the standard or portion of the standard in which the symbol is located or the symbol is not used according to the specifications for use of the symbol set forth in FDA's section 514(c) recognition, the explanatory text as provided ... Use of Symbols in Labeling: Frequently Asked Questions | FDA Manufacturers should look to the final rule, not the withdrawn 2004 guidance, when determining their use of symbols in new labeling and when making labeling updates. 14.

















.png.aspx)





Post a Comment for "43 fda guidance use of symbols on labels"