42 fda approved health claims on food labels
'FDA Cleared' vs. 'FDA Approved': What's the Difference? In short, "FDA Approved" and "FDA Cleared" are both valid labels to see on medical supplies; while "FDA Registered" and "FDA Certified" are not. And if you want to make sure a certain item ... Labeling and Label Approval | Food Safety and Inspection Service Labeling and Label Approval FSIS develops and provides labeling guidance, policies and inspection methods and administers programs to protect consumers from misbranded and economically adulterated meat, poultry, and egg products which ensure that all labels are truthful and not misleading.
FDA Food Product Labeling & Packaging Requirements - ESHA The Nutrition Facts Label is used to communicate important information about the food consumers eat. The FDA also governs what label format to use on your product based on package size and contents. The Nutrition Facts Label must show: Serving size ( Consult the RACC to determine this) Household measure/common household unit Servings per container

Fda approved health claims on food labels
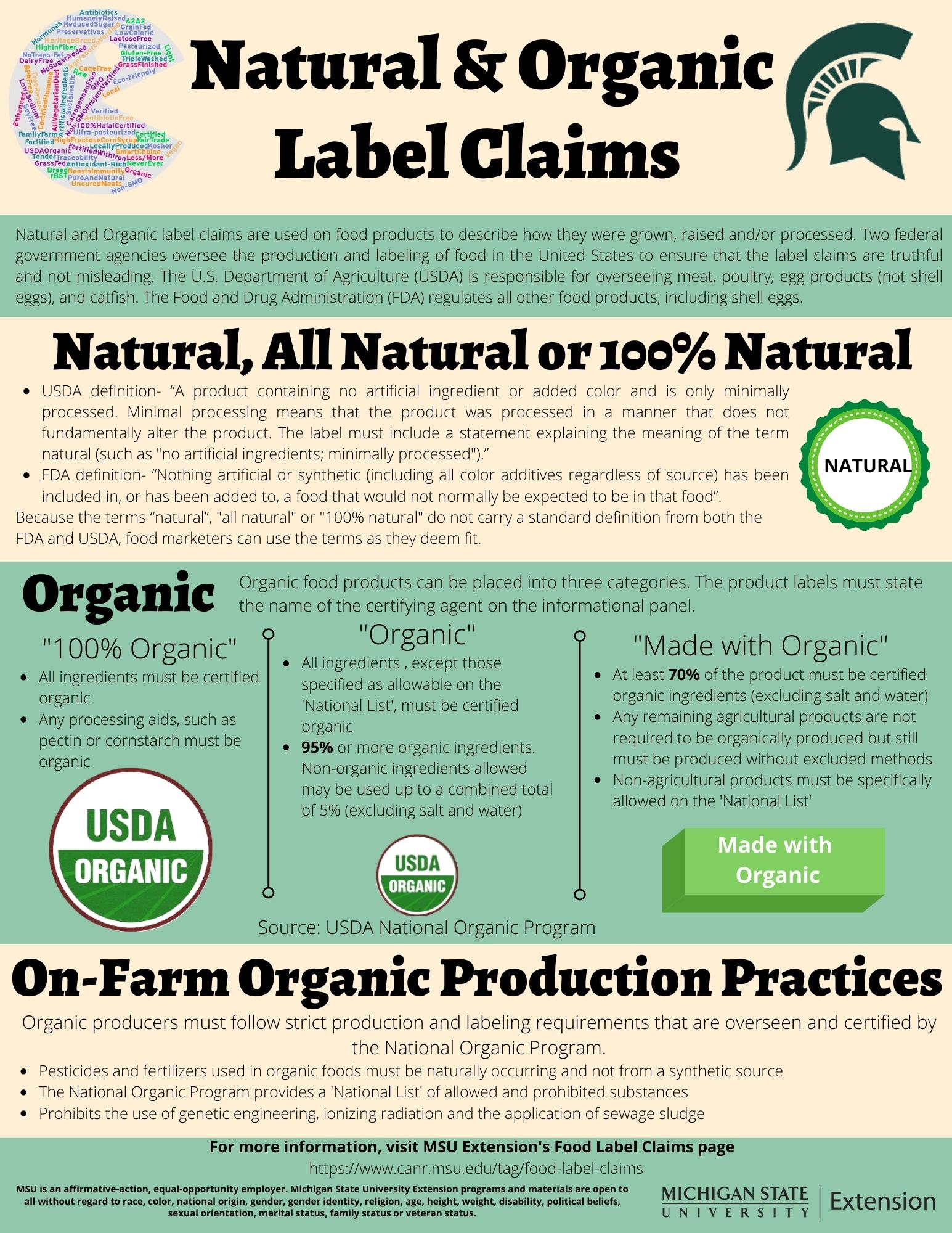
The Legality of Food Labeling Claims: FDA's Regulations - National ... Although food labeling claims can be helpful for consumers seeking certain qualities in the food they buy, sometimes labeling claims can mislead consumers. ... any food manufacturer can use a previously approved qualified health claim if they meet the conditions in the letter. ... To learn more about food labeling, read FDA's Food Labeling ... The Legality of Food Labeling Claims: Eggs and Dairy - National ... A "labeling claim" refers to a term on a label that is not required, but the manufacturer has chosen to include it—usually for marketing purposes. However, if a manufacturer includes a labeling claim, they usually must follow certain federal food labeling regulations. The type of food will determine which agency regulates the claims. Qualified Health Claims: Letters of Enforcement Discretion | FDA Letter Updating the Qualifying Level of Oleic Acid for the Oleic Acid From Edible Oils and Coronary Heart Disease (Corbion Biotech Petition) Qualified Health Claim July 16, 2020. Folic Acid ...
Fda approved health claims on food labels. Food Labels Guide & Examples | How to Read Nutrition Labels - Video ... Health claims must be based on scientific evidence to be approved by the FDA. Health claims on food labels can be exemplified in the following scenarios: Macadamia nuts reduce the risk of coronary ... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,... Authorized Health Claims That Meet Significant Scientific Agreement Authorized Health Claims That Meet the Significant Scientific Agreement (SSA) Standard Authorized health claims in food labeling are claims that have been reviewed by FDA and are allowed on food... Labeling Policies | Food Safety and Inspection Service Final rule amending the nutrition labeling regulations to change the definition of "meal-type" products to allow for nutrient content claims on multiple-serve food containers, to adopt the definition of "main dish" used by the Food and Drug Administration (FDA), and to define how meal-type products and main dishes should be nutrition labeled.
Health Claims on Food Labels | LegalMatch Do Health Claims Have to Be Authorized by the FDA? In short, yes. A health claim must be approved by the Food and Drug Administration ("FDA") before the manufacturer is allowed to put the health claim on one of their food products. In general, there are two ways in which a manufacturer can obtain FDA approval: CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (f) Model additional health claims for calcium and vitamin D. The following model health claims may be used in food labeling to describe the relationship between calcium, vitamin D, and... Qualified Health Claims | FDA - U.S. Food and Drug Administration Food manufacturers can petition the agency to consider exercising enforcement discretion for the use of a qualified health claim. The FDA does not "approve" qualified health claim petitions. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Sec. 101.81 Health claims: Soluble fiber from certain foods and risk of coronary heart disease (CHD). (a) Relationship between diets that are low in saturated fat and cholesterol and that include...
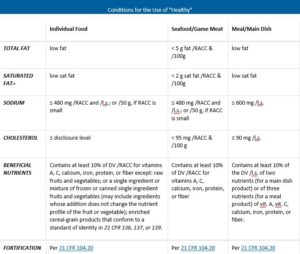
13 Misleading Food Label Claims and How Not to Be Tricked - Sentient Media To use the label low-calorie in the United States, the FDA mandates that foods contain 40 calories or less per reference amount customarily consumed ( RACC ). Meals and main dishes should include 120 calories or less per 100 grams of food. 6. Label Says "Low-Carb" The FDA does not have any guidelines for the labeling of foods as low-carb. CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) a claim that a specific ingredient or food component is absent from a product, provided that the purpose of such claim is to facilitate avoidance of the substances because of food allergies... Nutrient Content Claims | FDA - U.S. Food and Drug Administration Nutrient Content Claims. See Claims That Can Be Made for Conventional Foods and Dietary Supplements for definitions of claims. Final Rule: Food Labeling: Nutrient Content Claims; Alpha-Linolenic ... The FDA considers a "healthy" food label - Axios The FDA itself is in the process of updating its definition, which dates back to 1994. Other concerns are that such a label could be of dubious value, used too liberally by food makers, or seen by consumers as a product endorsement by the FDA. Nutritionists make the point that a balanced diet matters more to health than any individual food.
Label Submission and Approval System (LSAS) | Food Safety and ... Who do I contact if I need assistance or have a technical issue concerning LSAS? The LSAS administrator will be your first contact. Email the administrator at LSAS@usda.gov or call 301-504-0837. We ask that you use these resources first rather than calling the main Labeling and Program Delivery Division (LPDD) lines so that we can track all issues and provide appropriate resolutions.
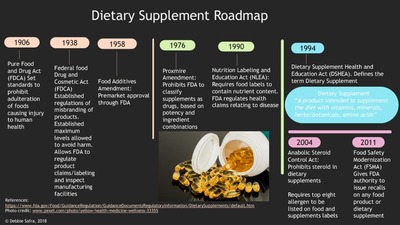
Making Health Claims: Follow the Science, Not the Hype - Food Processing These 12 food/disease connections are the only claims that the FDA has determined, in the 32 years of the labeling act, to be truly backed by "the totality" of scientific evidence. While those 12 are the only authorized health claims, there is another category of health claims called "qualified."
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and...
Label Claims for Conventional Foods and Dietary Supplements there are three ways in which fda exercises its oversight in determining which health claims may be used on a label or in labeling for a conventional food or dietary supplement: 1) the 1990...
The Legality of Food Labeling Claims: FSIS's Regulations for Meat and ... Health claims and structure and function claims on FSIS regulated food products are the same as those claims on FDA regulated products. Health claims characterize a relationship between a nutrient and a reduced risk of disease or a health-related condition. 21 C.F.R. § 101.14(a)(1). Structure and function claims are similar to health claims ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The following model health claims may be used in food labeling to describe the relationship between noncariogenic carbohydrate sweetener-containing foods and dental caries. (1) Examples of the full...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration PART 101 -- FOOD LABELING Subpart E - Specific Requirements for Health Claims Sec. 101.83 Health claims: plant sterol/stanol esters and risk of coronary heart disease (CHD). (a) Relationship...
8 misleading food marketing labels | AGDAILY It turns out that many, if not most of them, really aren't. Here are 8 of the most common misleading food marketing claims: 1. No nitrites or nitrates added. Although this particular labeling regulation may be changing soon, you may have noticed the "No Nitrites or Nitrates Added" label on processed meat products, such as deli meats and ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration The label or labeling of food may contain a folate/neural tube defect health claim provided that: (1) General requirements. The health claim for a food meets all of the general requirements of §...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements...
Qualified Health Claims: Letters of Enforcement Discretion | FDA Letter Updating the Qualifying Level of Oleic Acid for the Oleic Acid From Edible Oils and Coronary Heart Disease (Corbion Biotech Petition) Qualified Health Claim July 16, 2020. Folic Acid ...
The Legality of Food Labeling Claims: Eggs and Dairy - National ... A "labeling claim" refers to a term on a label that is not required, but the manufacturer has chosen to include it—usually for marketing purposes. However, if a manufacturer includes a labeling claim, they usually must follow certain federal food labeling regulations. The type of food will determine which agency regulates the claims.
The Legality of Food Labeling Claims: FDA's Regulations - National ... Although food labeling claims can be helpful for consumers seeking certain qualities in the food they buy, sometimes labeling claims can mislead consumers. ... any food manufacturer can use a previously approved qualified health claim if they meet the conditions in the letter. ... To learn more about food labeling, read FDA's Food Labeling ...
































Post a Comment for "42 fda approved health claims on food labels"